Abstract
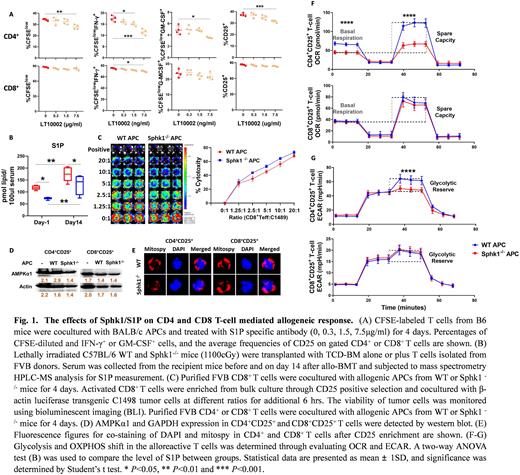
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative option for the treatment of hematological malignancies. Patients who undergo allo-HCT can benefit from graft-versus-leukemia (GVL) effects, while graft-versus-host disease (GVHD) remains the significant cause of morbidity and mortality after transplantation. Sphingosine-1-phosphate (S1P) signaling involves many biogenetic processes of different immune cells. Patients with acute GVHD exhibit a significantly higher level of S1P, as compared to the patients without GVHD. Donor infiltrated T cells play a vital role in regulating the GVH and GVL responses. In a mixed lymphocyte assay in vitro, we found that specific blockage of S1P obviously attenuated the alloreactivities of CD4+ T cells while allogeneic CD8+ T cells were not sensitive to the S1P signal (Fig. 1A). Therefore, we investigated the impact of targeting S1P/S1PR signaling on GVH and GVL responses and underlying mechanisms in current research.
We first observed that serum levels of S1P were significantly higher in the mice after allogeneic bone marrow transplantation (allo-BMT) than before BMT mouse (Figure 1B). The secreted S1P is mainly synthesized from sphingosine by sphingosine kinase 1 (Sphk1). Compared to WT counterparts, Sphk1-/- mice reduced serum S1P levels before or after allo-BMT (Fig. 1B). In the MHC-mismatched BMT model, Sphk1/S1P deficiency significantly improved GVHD due to the impairment of migration and cytokine production of CD4+ T cells but it did not impact the alloresponse of CD8+ T cells. After being stimulated with allogeneic WT or Sphk1-/- APCs, CD8+ T cells obtained comparable CTL activities (Fig. 1C). Mechanically, the alloreactive CD4+ T cells decreased the expression of AMPKα1 (Fig. 1D) and phosphorylation of its downstream responder AKT, and subsequently mitochondrial content (Fig. 1E) and metabolism (Fig. 1F) upon Sphk1-/- APC stimulation (Figure D-G). However, OXPHOS, glycolysis and mitochondrial mass of CD8+ T cells were not impacted through a S1P-dependent AMPK-AKT signaling pathway (Fig. 1 D-G), which likely contributed to the maintenance of the GVL effect. In parallel, we found that S1PR1 is required for optimal T-cell pathogenicity to induce GVHD while dispensable for the GVL effect.
Collectively, our study provides a novel mechanistic insight into how the S1P signal differently modulates the alloresponse of CD4+ and CD8+ T cells and validates Sphk1/S1P as a therapeutic target for the prevention of GVHD while maintaining that GVL activity, which would benefit allo-HCT patients with hematologic malignancy.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal